Chemistry
Explore Chapters
Chemical Reaction are represented in symbolic form by a Chemical equation. The reactants and the products are written in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. In this section of Chemical Reactions Advantages of using a chemical reaction, Balanced and Unbalanced Chemical Equation, Change in Colour, Temperature, Characteristics of chemical Reactions, Chemical changes, Chemical Equation, Corrosion, Endothermic and Exothermic Reaction, Evolution of gas, Formation of precipitate, How to make equations more informative, Information conveyed by a chemical equation, Limitations of chemical Reaction, Physical Change, Physical State, Product, Reactant, Types of changes, , etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionMOCK (F) - 10th - Chemistry [Chemical Reactions I, Chemical Reactions II]ChemistryChemical Reactions I, Chemical Reactions II10 / 10Tutorial - 10th - Chemistry [Chemical Reactions I]ChemistryChemical Reactions I10 / 10View All FREE Test(s)
Chemical Reaction are represented in symbolic form by a Chemical equation. The reactants and the products are written in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. In this section of Chemical Reactions Combination Reaction, Decomposition Reaction, Displacement Reaction, Quicklime and Slaked Lime, Rancidity, Redox Reaction, Representation of Chemical Reaction, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionMOCK (F) - 10th - Chemistry [Chemical Reactions I, Chemical Reactions II]ChemistryChemical Reactions I, Chemical Reactions II10 / 10Tutorial - 10th - Chemistry [Chemical Reactions II]ChemistryChemical Reactions II10 / 10View All FREE Test(s)
An acid and a base are substances in chemistry. The aqueous solution of acid is sour in taste, it turns blue litmus red and neutralizes bases. Whereas aqueous solution of base is bitter in taste. It turns red litmus blue or neutralizes acids. salts are ionic compounds that result from the neutralization reaction of an acid and a base. In this section of Acids, Bases and Salts Beet root Solution, Indicator prepared from Red Cabbage Leaves, Chemical Properties of Acids and Bases, Common Acids and Bases used in Laboratory, Concentrated and Dilute acids, Definition of Acids and Bases, Indicators, Llitmus and Dry Turmeric Indicator, Methyl Orange and Phenolphthalein Indicators, Olfactory Indicators, Organic and Inorganic Acids, Oxo acids and Hydrated Acids, Reaction of Acids and Bases, Reaction of Metals with Acids and Bases, Strength of Acids and Bases, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Acids Bases and Salts I]ChemistryAcids Bases and Salts I10 / 10View All FREE Test(s)
An acid and a base are substances in chemistry. The aqueous solution of acid is sour in taste, it turns blue litmus red and neutralizes bases. Whereas aqueous solution of base is bitter in taste. It turns red litmus blue or neutralizes acids. salts are ionic compounds that result from the neutralization reaction of an acid and a base. In this section of Acids, Bases and Salts Acids and Physical Properties of Acids, Bases and Physical Properties of Bases, Chlor Alkali Process, Classification of Salts, Common Salts in Daily Life, General Properties of Salts, Importance of pH in Daily Life, Important Chemical Compounds, pH of Salts, Preparation of Salts, Salts, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Acids Bases and Salts II]ChemistryAcids Bases and Salts II10 / 10View All FREE Test(s)
Materials can be divided into metals and nonmetals. In this section of Metals and Non Metals Metal React With Water, Metals and Non-Metals, Metals Burnt in Air, Metals React With Acids, Metals React With Other Metal Salts, Physical Property of Metal - Conductivity, Density, Ductility, Hardness, Physical Property of Metal - Melting Point and Boiling Point, Physical Property of Metal - Physical State, Physical Property of Metal - Sonority, Physical Property of Metal Appearance, Physical Property of Non Metal, , etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Metals and Non Metals I]ChemistryMetals and Non Metals I10 / 10View All FREE Test(s)
Materials can be divided into metals and nonmetals. In this section of Metals and Non Metals Alloys and its Importance, Conductivity of Ionic Compounds, Corrosion and its Prevention, Different types of ores, Enrichment or Dressing of an Ore, Extraction of metals at the bottom of the Activity Series of High Activity, Extraction of metals at the bottom of the Activity Series of Low Activity, Extraction of Metals at the middle of the activity Series of Medium Reactivity, Melting and Boiling Point of Ionic Compounds, Metallurgical Operations, Metallurgy and its Principles, Mineral and Ores, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Metals and Non Metals II]ChemistryMetals and Non Metals II10 / 10View All FREE Test(s)
The element Carbon is a nonmetal, its symbol is C. The atomic number of carbon is 6 mass number is 12. In this section of Carbon and Its Compounds Alkanes, Alkenes, Alkynes, Allotropes of Carbon, Carbon - As an Element, Covalent and Ionic Bonds, Diamonds, Differences between Organic and Inorganic Compounds, Fullerene, Graphite, Hydro-Carbons, Organic Chemistry, Property of Carbon Compounds- Bond Strength, Property of Carbon Compounds- Catenation, Property of Carbon Compounds- Isomerism, Property of Carbon Compounds- Multiple Bond Formation, Property of Carbon Compounds- Tetravalency, Saturated and Unsaturated Hydro Carbons, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Carbon and Its Compounds I]ChemistryCarbon and Its Compounds I10 / 10View All FREE Test(s)
The Chemistry of Carbon Compound is called Organic Chemistry. Carbon forms a large number of compounds. In this section of Carbon and Its Compounds Addition Reaction, Alcohol Functional Group, Aldehyde Functional Group, Carboxylic Acid Functional Group, Effect of Ethanol on Human Beings, Esterification and Saponification, Ethanol, Ethnoic Acid, Flames, Fossil Fuels, Functional Groups, Halogens Functional Group, Homologous Series, Ketone Functional Group, Nomenclature of Carbon Compounds, Oxidation of Carbon Compounds, Soaps and Detergents, Sources of Organic Compounds, Subsitution Reaction of Carbon Compounds, etc. have been explained. Watch the videos and master the concepts
Scientist have discovered 111 chemical elements till now. In the long form Periodic table the elements are arranged in the order of their atomic numbers. Atomic number of an element is equal to the number of protons inside the nucleus of its atom. In this section of Periodic Classification Achievements and Limitations of Mendeleev Periodic Table, Dobreiner Triads, Earlier Attempts of Classification of Elements, Mendeleev Periodic Table, Newland Law of Octave, Periodic Classification, , etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Periodic Classification I]ChemistryPeriodic Classification I10 / 10View All FREE Test(s)
Scientist have discovered 111 chemical elements till now. In the long form Periodic table the elements are arranged in the order of their atomic numbers. Atomic number of an element is equal to the number of protons inside the nucleus of its atom. In this section of Periodic Classification Metals and Non-Metals in Periodic Classification, Modern Periodic Table, Position of Elements in the Modern Periodic Table, Trends in the Modern Periodic Table, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Periodic Classification II]ChemistryPeriodic Classification II10 / 10View All FREE Test(s)
This branch of Chemistry refers to the sustainable utilization of major natural resources, such as land, water, air, minerals, forests, fisheries, and wild flora and fauna. These resources provide the ecosystem services for better quality to human life. In this section of Management of Natural Resources, Conservation of Soil and Water Resources, Forest and Wild Life Resources, Ganga Action Plan, Global Warming, Greenhouse Effect, Management and Conservation of Natural Resources, Management of Natural Resources, Renewable and Non Renewable Resources, Sustainability of Natural Resources, The Chipko Movement, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Management of Natural Resources I]ChemistryManagement of Natural Resources I10 / 10View All FREE Test(s)
This branch of Chemistry refers to the sustainable utilization of major natural resources, such as land, water, air, minerals, forests, fisheries, and wild flora and fauna. These resources provide the ecosystem services for better quality to human life. In this section of Management of Natural Resources, Big Dams, Coal and Petroleum, Harvesting of Water, Legal Measure to Save The Environment, Save The Narmada Movement, The Adverse Effects of a Dam, Water As A Resource, Watershed Management, etc. have been explained. Watch the videos and master the concepts
- Sample Test(s)Test NameSubject(s)Topics / ChaptersQuestions / MarksActionTutorial - 10th - Chemistry [Management of Natural Resources II]ChemistryManagement of Natural Resources II10 / 10View All FREE Test(s)
Explore Concepts (Click & View)
- Types of changes
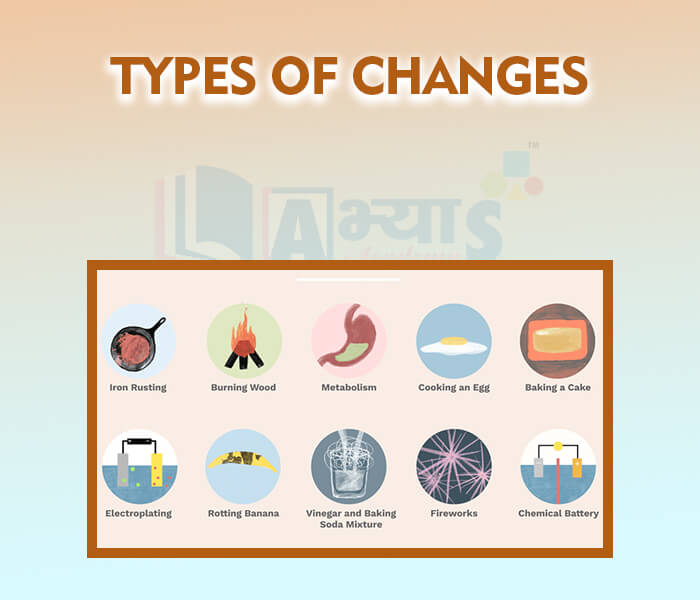
- Physical Change

- Chemical changes
- Chemical Reaction

- Reactant
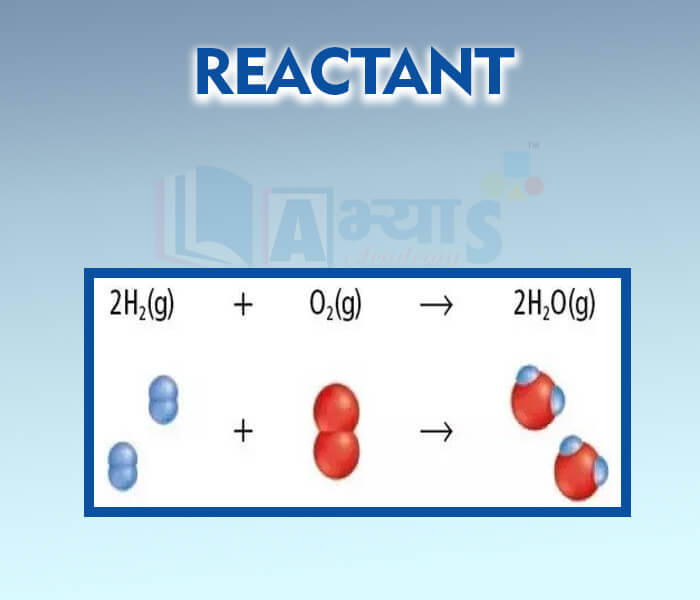
- Product

- Characteristics of chemical Reactions
- Evolution of gas

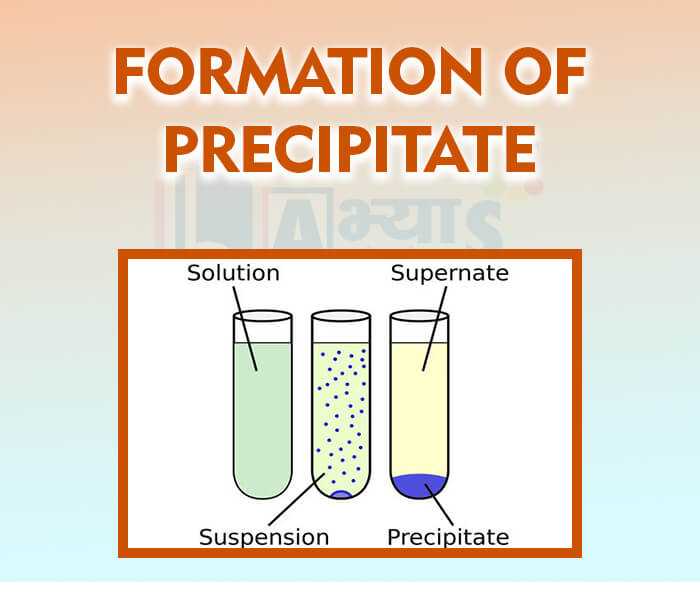
- Formation of precipitate
- Change in Temperature

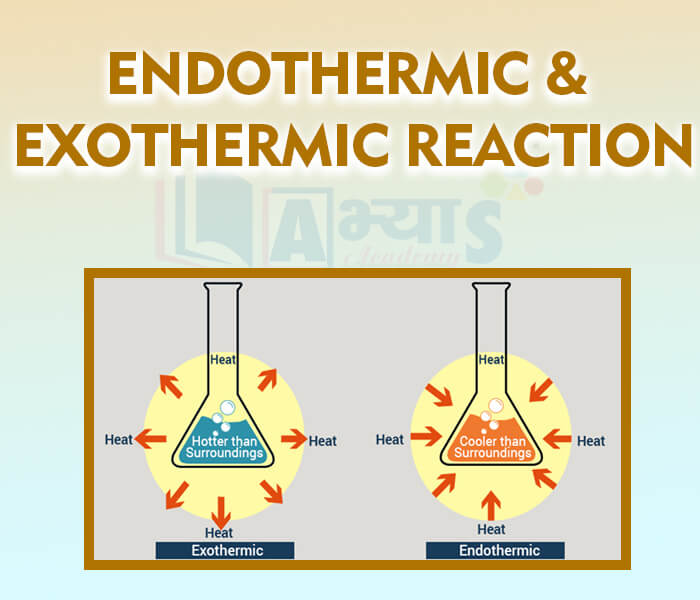
- Endothermic and Exothermic Reaction
- Change in Colour

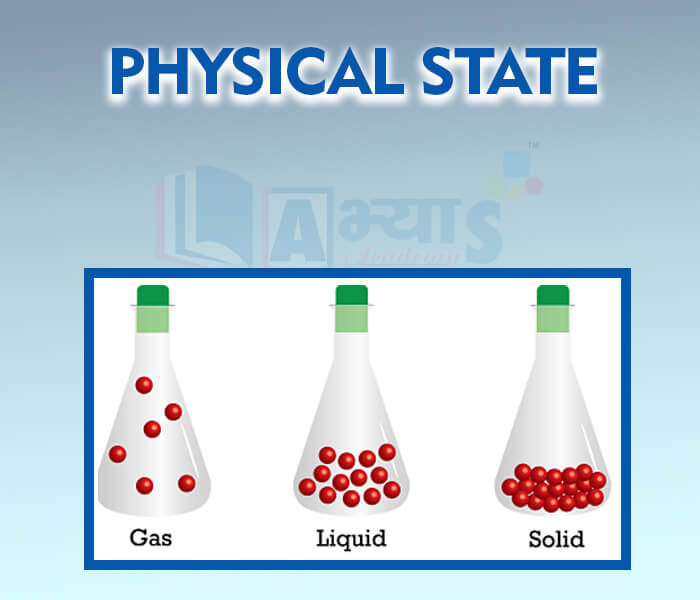
- Physical State
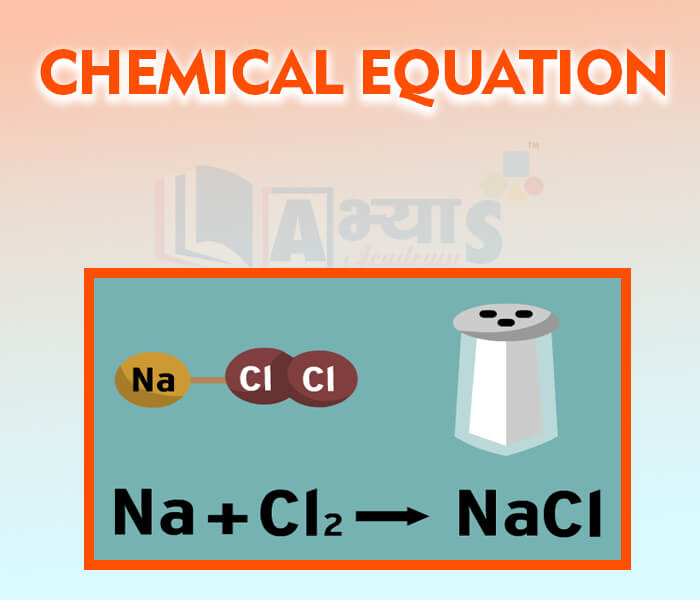
- Chemical Equation
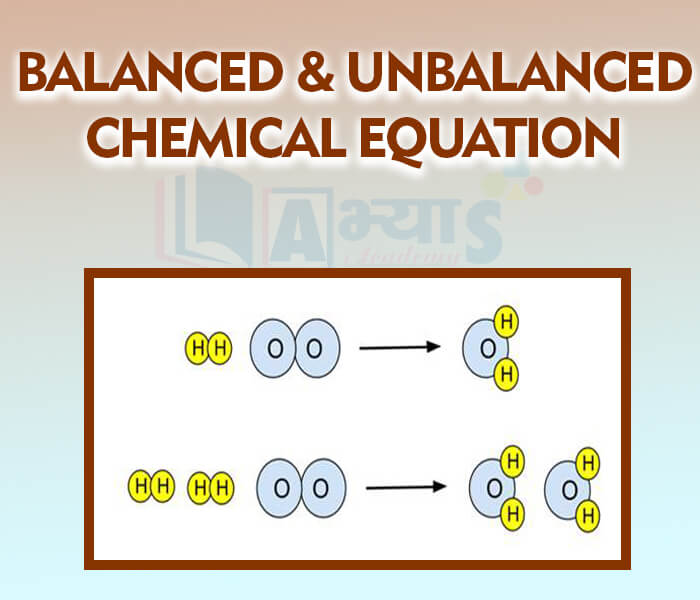
- Balanced and Unbalanced Chemical Equation
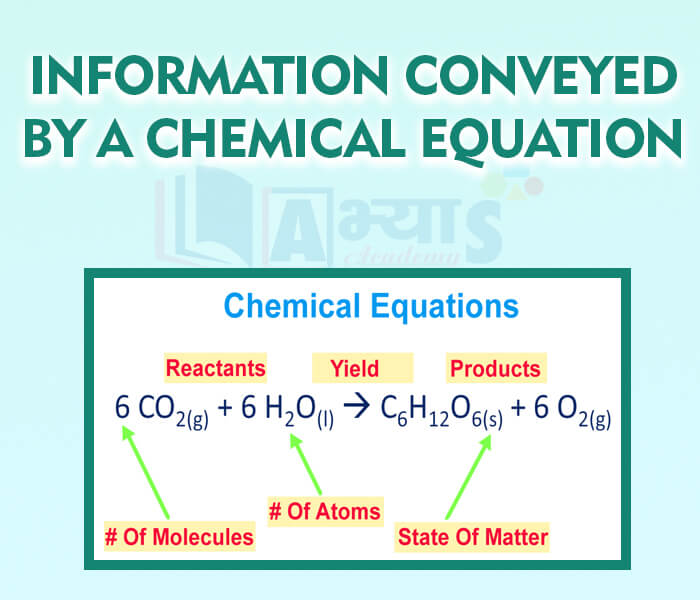
- Information conveyed by a chemical equation
- Advantages of using a chemical reaction
- Limitations of chemical Reaction
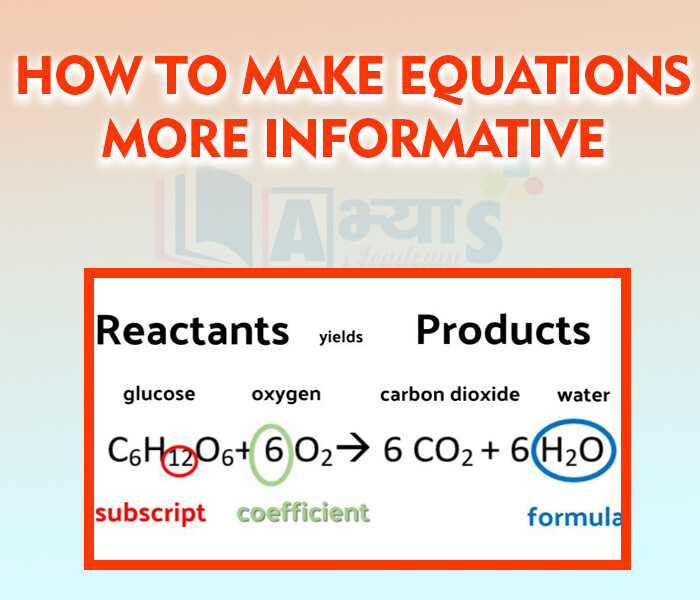
- How to make equations more informative
- Corrosion

Explore Concepts (Click & View)
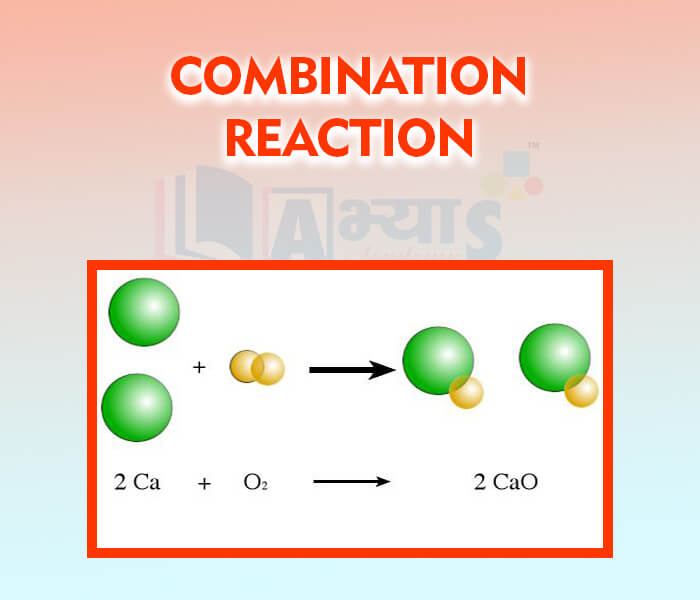
- Combination Reaction
- Representation of Chemical Reaction
- Decomposition Reaction

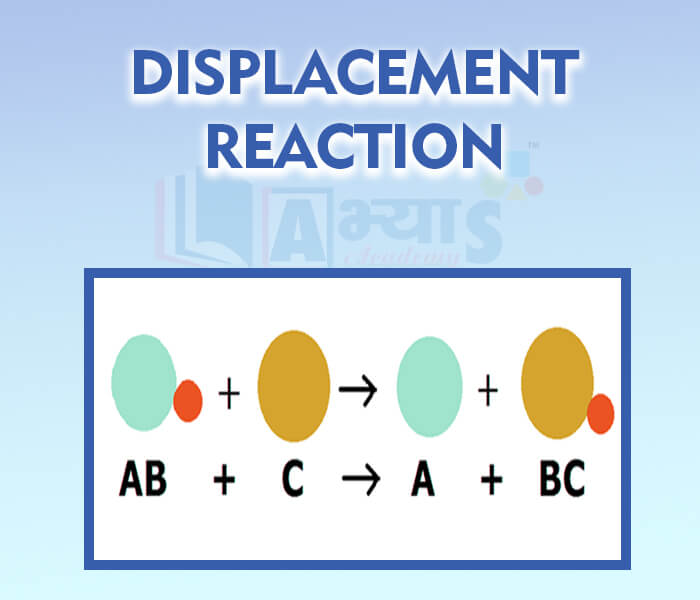
- Displacement Reaction
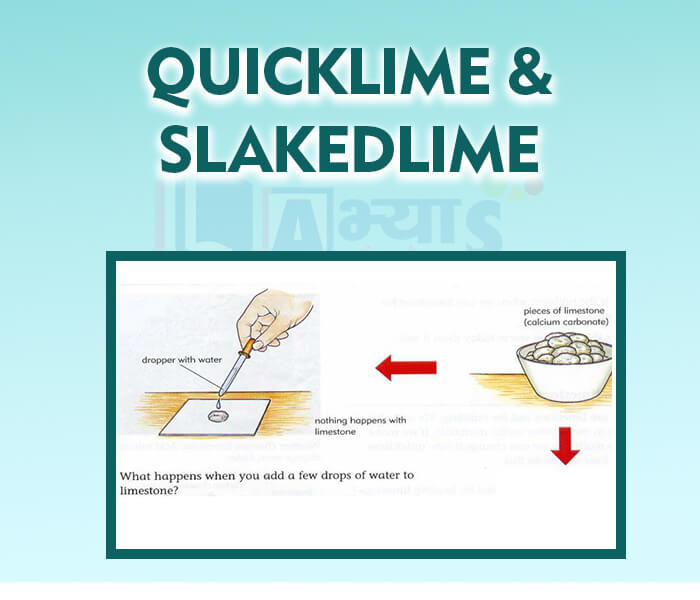
- Quicklime and Slaked Lime
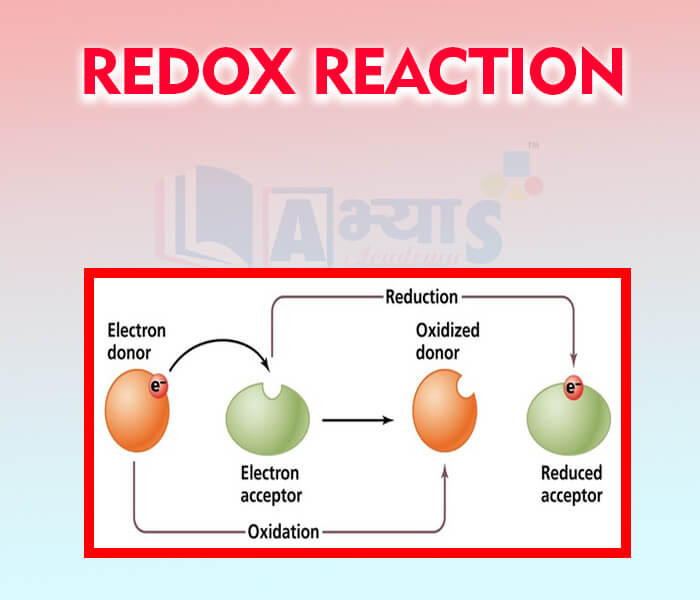
- Redox Reaction
- Rancidity

Explore Concepts (Click & View)
- Definition of Acids and Bases

- Acids and Physical Properties of Acids

- Oxo acids and Hydrated Acids

- Organic and Inorganic Acids

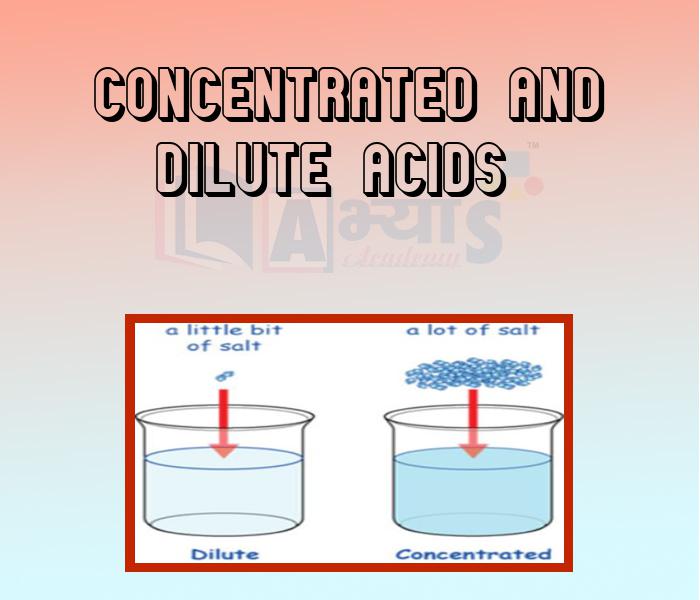
- Concentrated and Dilute acids
- Strong and Weak Acids

- Uses of Acids

- Uses of Bases

- Bases and Physical Properties of Bases
- Chemical Properties of Acids and Bases

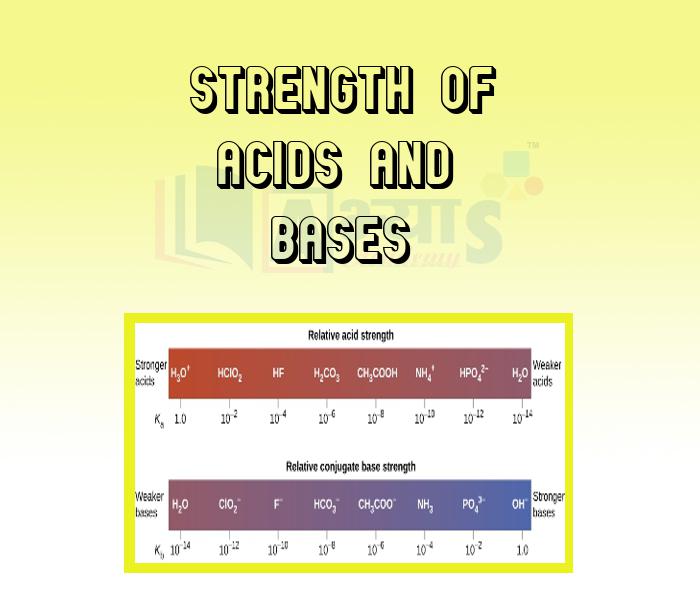
- Strength of Acids and Bases
- Indicators
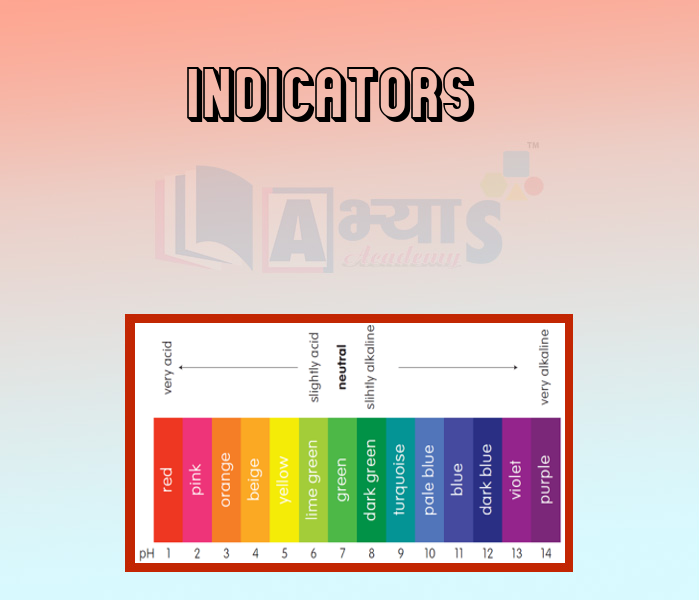
- Universal Indicators

- Reaction of Metals with Acids and Bases

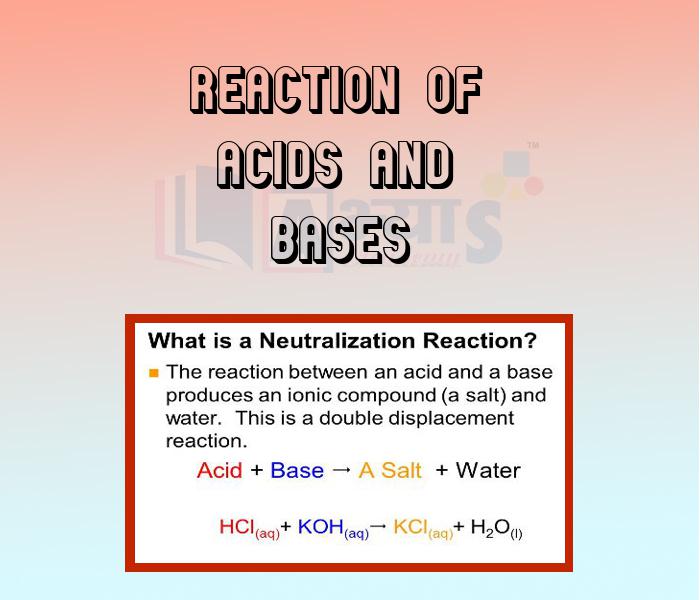
- Reaction of Acids and Bases
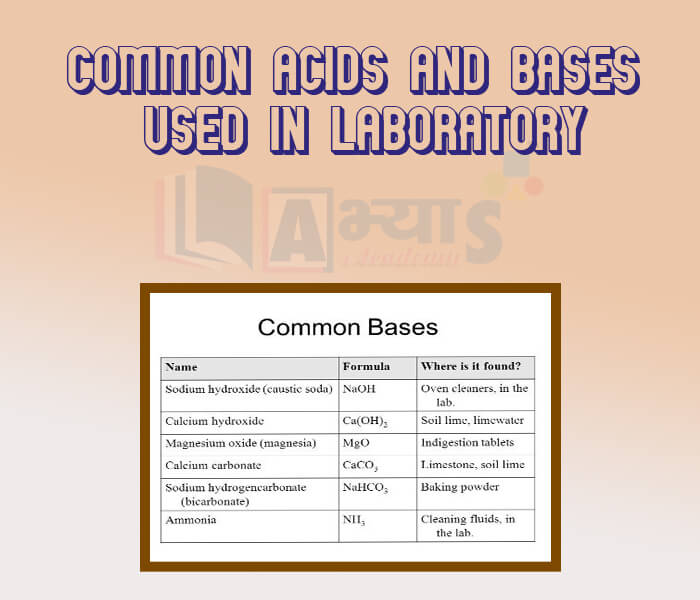
- Common Acids and Bases used in Laboratory
- Litmus and Dry Turmeric Indicator

- Methyl Orange and Phenolphthalein Indicators
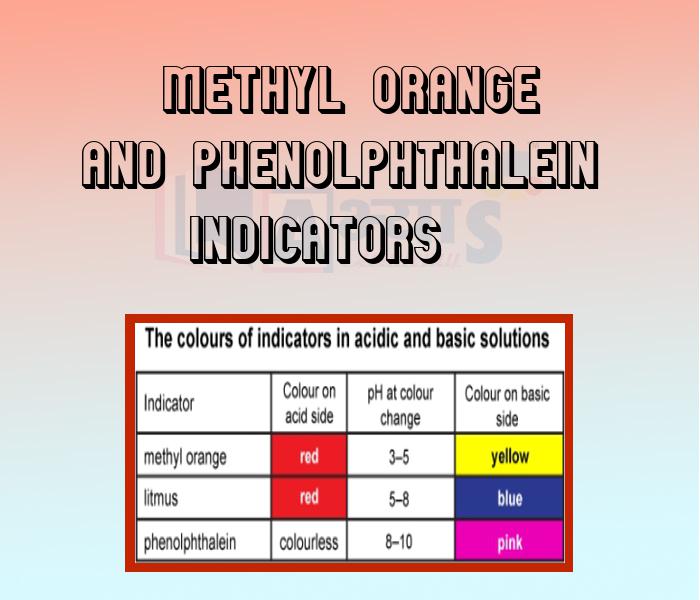
- Olfactory Indicators

- Beet root Solution, Indicator prepared from Red Cabbage Leaves

Explore Concepts (Click & View)
- Salts

- Classification of Salts

- Preparation of Salts

- General Properties of Salts

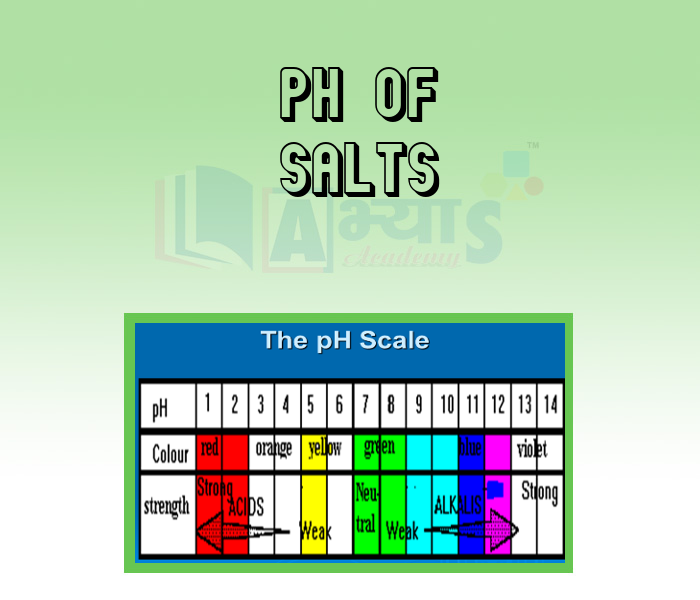
- pH of Salts
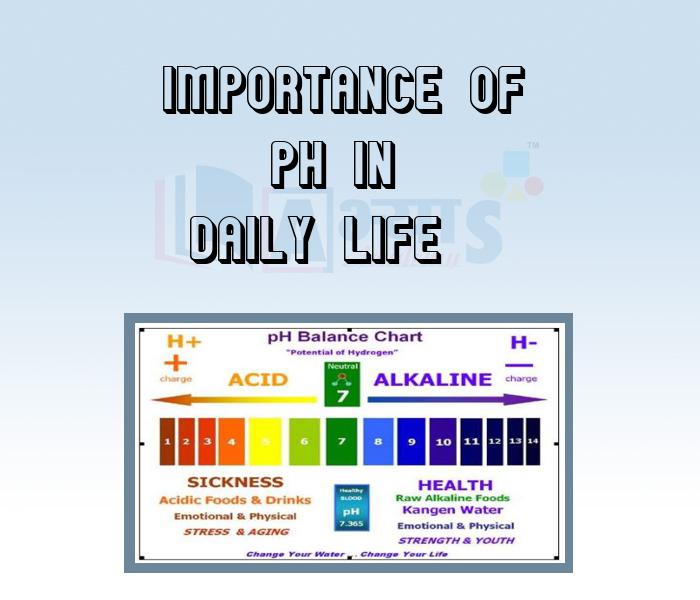
- Importance of pH in Daily Life
- Common Salts in Daily Life

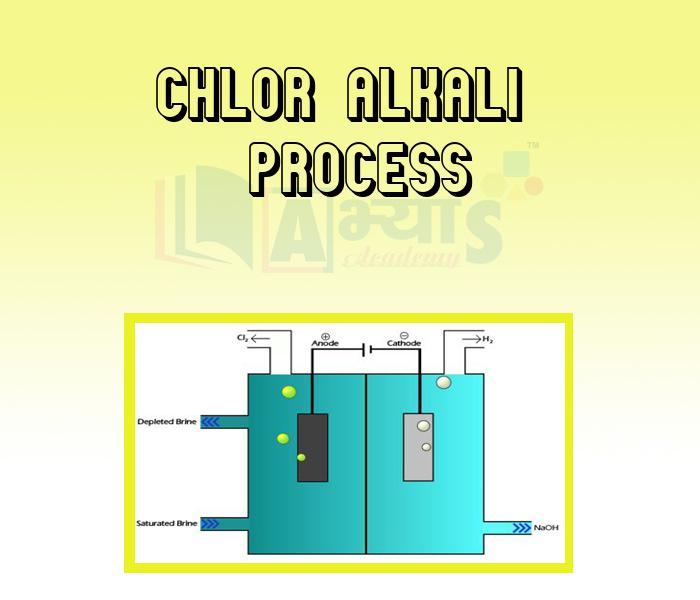
- Chlor Alkali Process
- Important Chemical Compounds

Explore Concepts (Click & View)
- Metals and Non-Metals

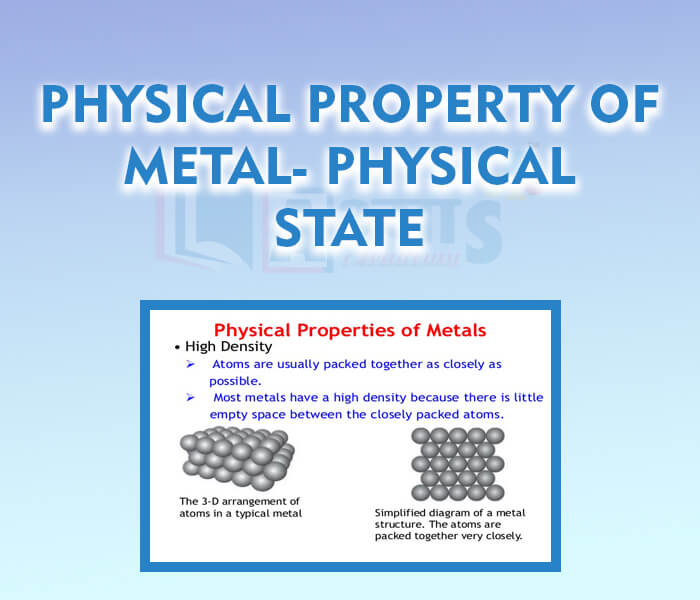
- Physical Property of Metal - Physical State
- Physical Property of Metal - Ductility

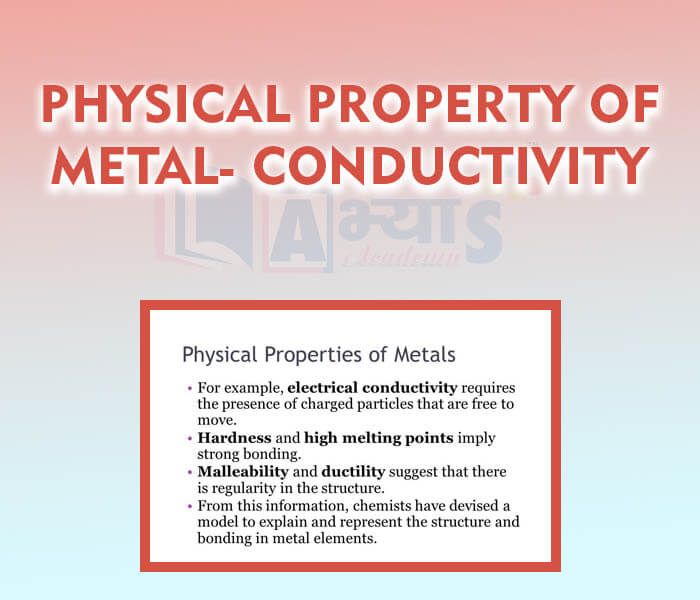
- Physical Property of Metal - Conductivity
- Physical Property of Metal Appearance

- Physical Property of Metal - Hardness

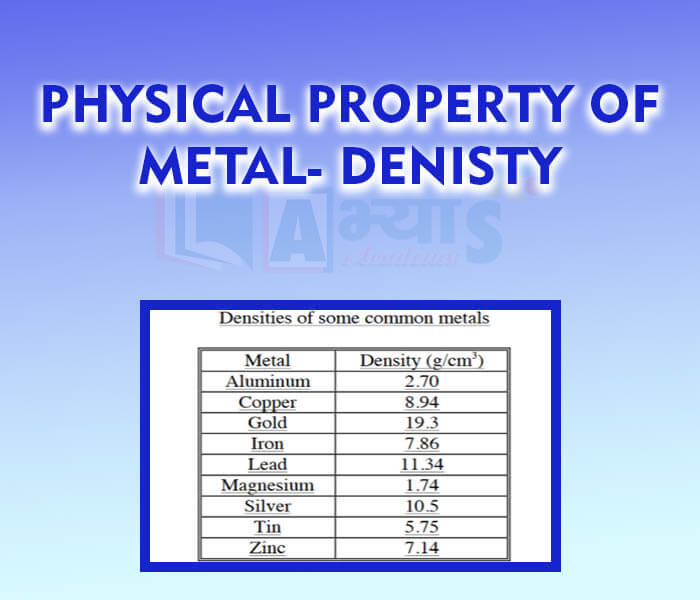
- Physical Property of Metal - Denisty
- Physical Property of Metal - Melting Point and Boiling Point

- Physical Property of Metal - Sonority

- Reactivity Series

- Physical Property of Non-Metal - Physical State

- Physical Property of Non-Metal - Conductivity

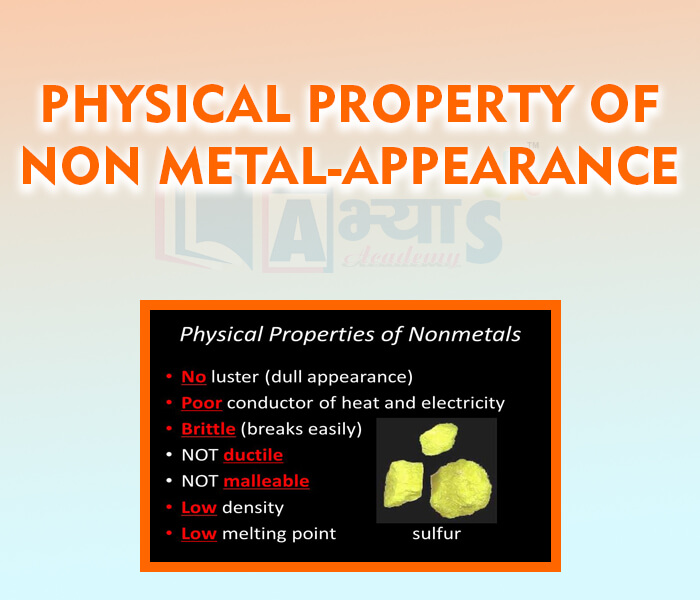
- Physical Property of Non-Metal - Appearance
- Physical Property of Non-Metal - Hardness

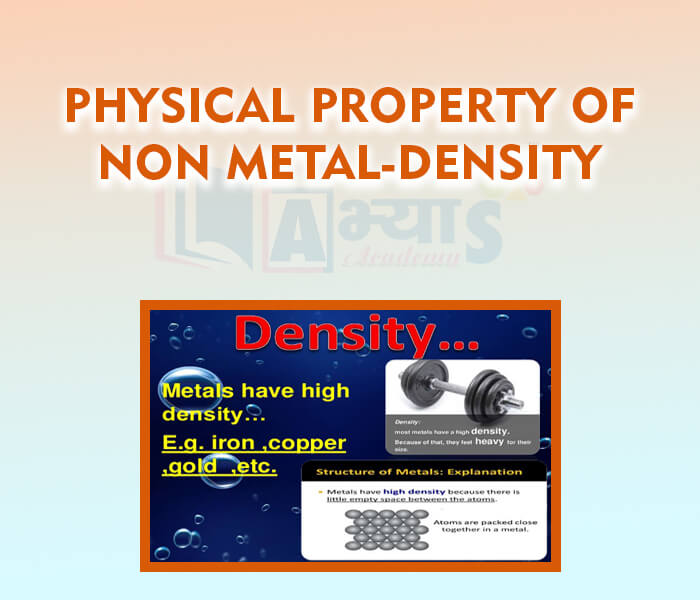
- Physical Property of Non-Metal - Density
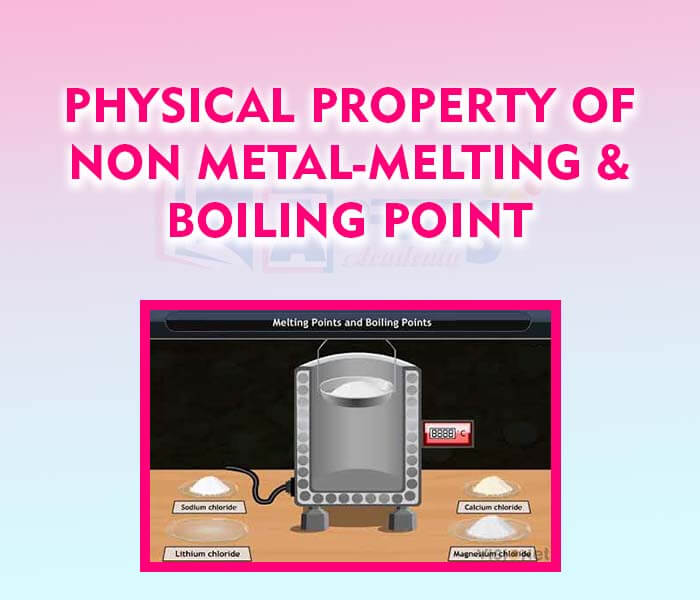
- Physical Property of Non-Metal - Melting and Boiling Point
- Physical Property of Non-Metal - Sonority

- Reaction with Oxygen

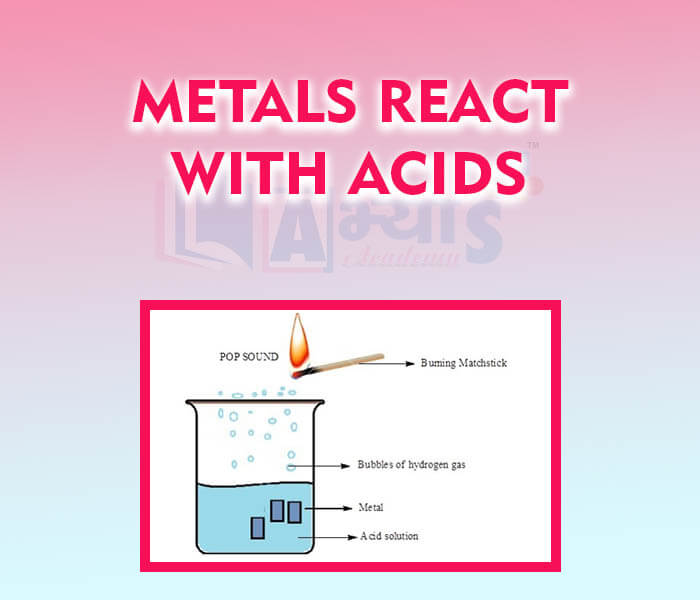
- Metals React With Acids
- Reaction With Water

- Uses of Metals

- Metals React With Other Metal Salts

- Uses of Non Metals

Explore Concepts (Click & View)
- Mineral and Ores

- Different types of ores

- Metallurgy and its Principles

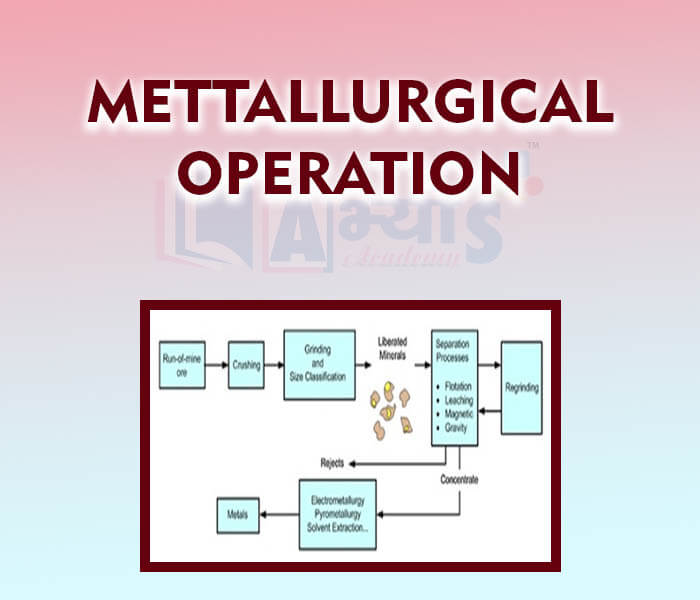
- Metallurgical Operations
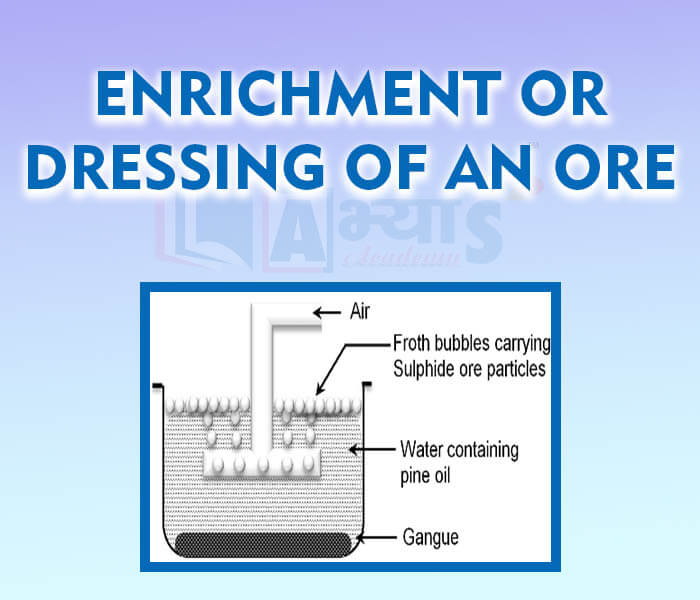
- Enrichment or Dressing of an Ore
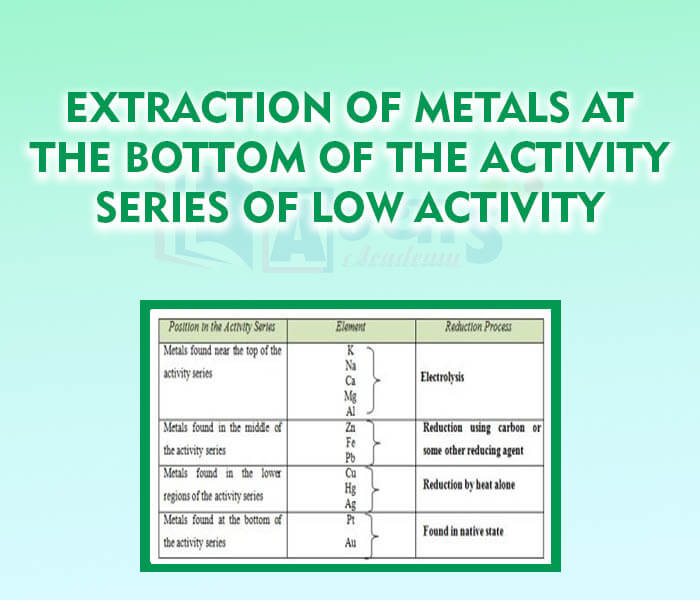
- Extraction of metals at the bottom of the Activity Series of Low Activity
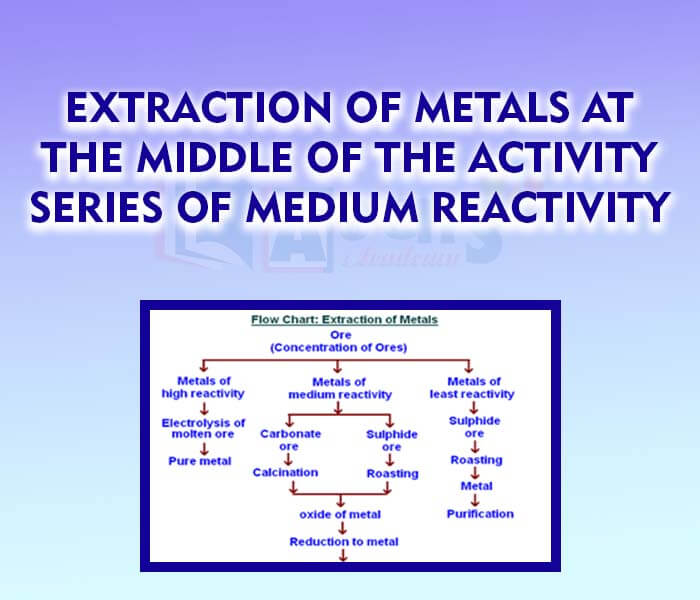
- Extraction of Metals at the middle of the activity Series of Medium Reactivity
- Extraction of metals at the top of the Activity Series of High Activity

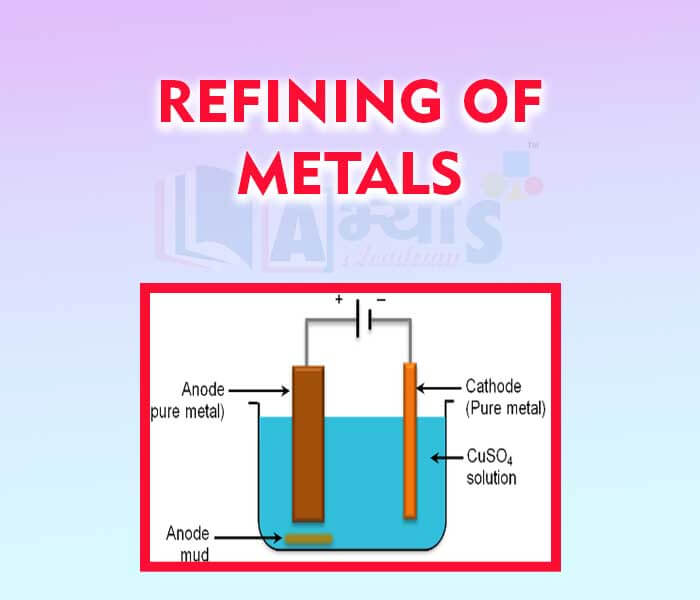
- Refining of Metals
- Corrosion and its Prevention

- Alloys and its Importance

- Physical Nature of Ionic Compounds

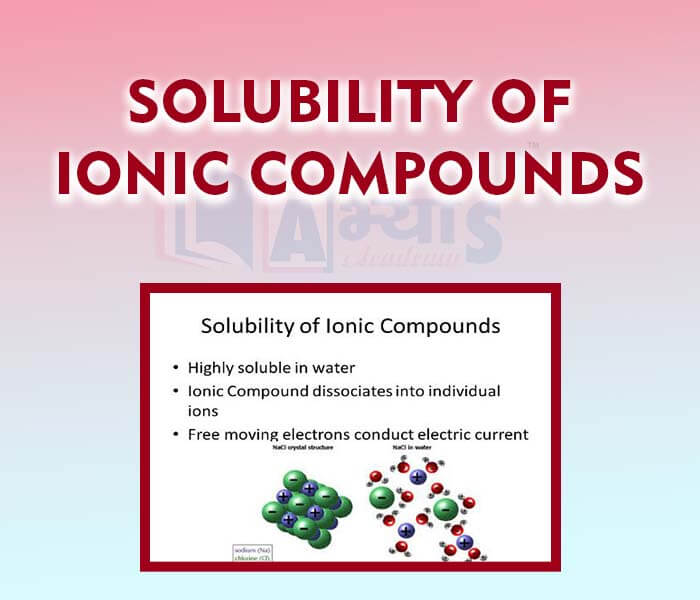
- Solubility of Ionic Compounds
- Melting and Boiling Point of Ionic Compounds
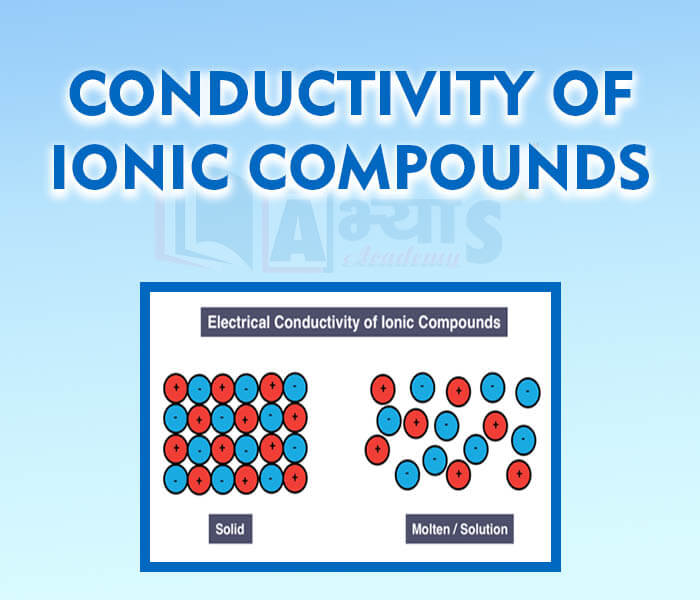
- Conductivity of Ionic Compounds
Explore Concepts (Click & View)
- Carbon - As an Element

- Covalent and Ionic Bonds

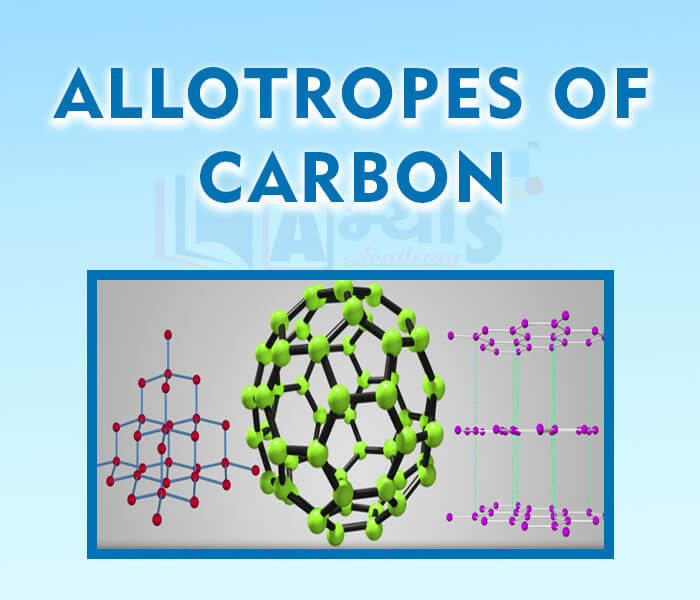
- Allotropes of Carbon
- Diamonds

- Graphite

- Fullerene
- Differences between Organic and Inorganic Compounds

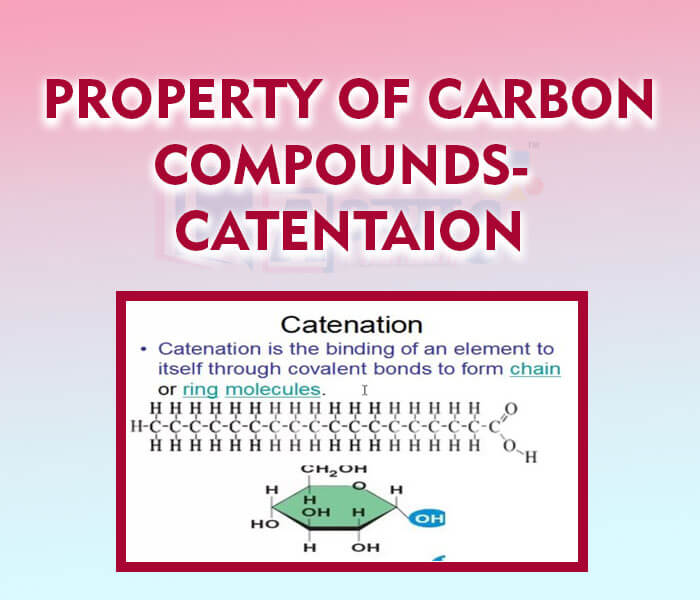
- Property of Carbon Compounds- Catenation
- Property of Carbon Compounds- Tetravalency

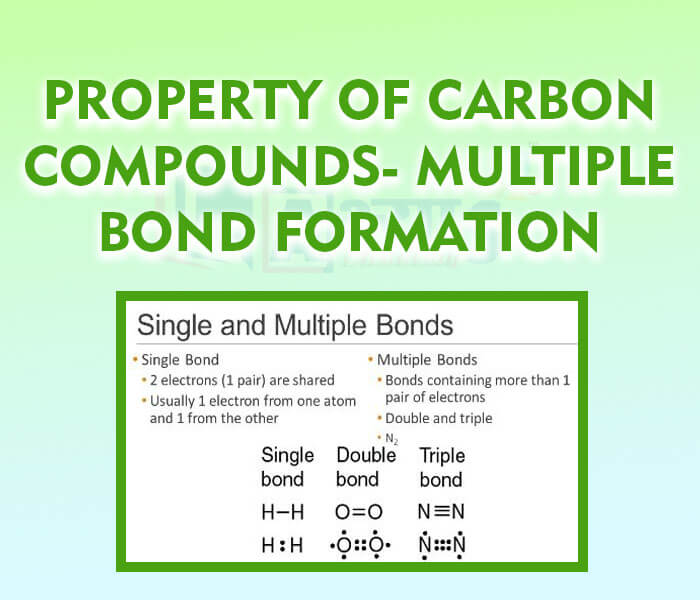
- Property of Carbon Compounds- Multiple Bond Formation
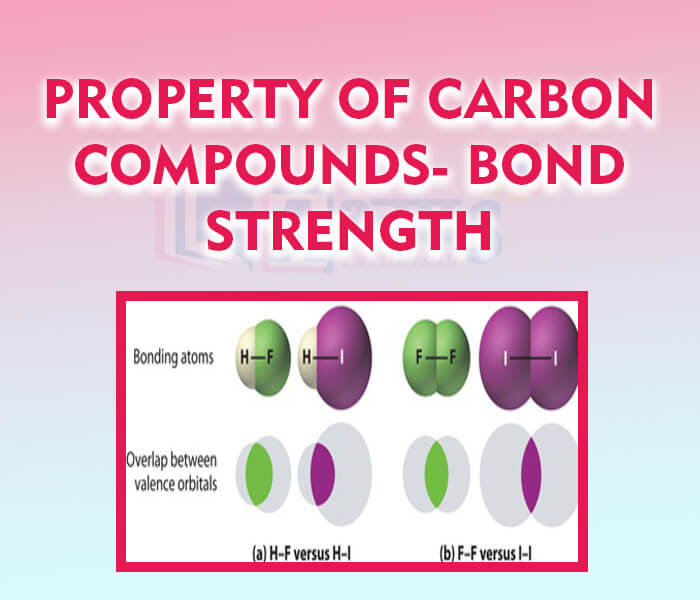
- Property of Carbon Compounds- Bond Strength
- Organic Chemistry
- Hydro-Carbons

- Saturated and Unsaturated Hydro Carbons
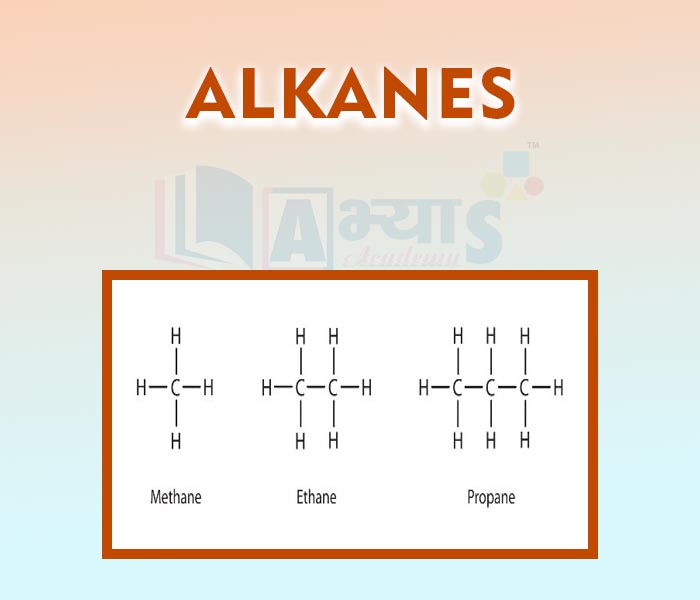
- Alkanes
- Alkenes
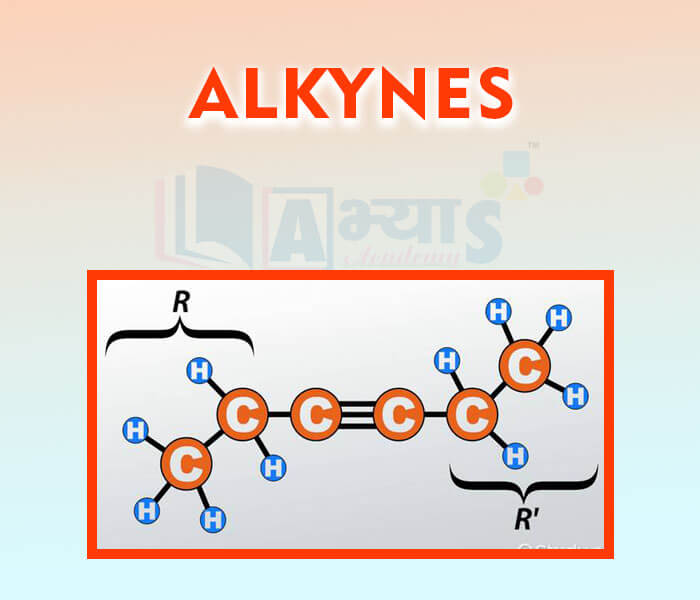
- Alkynes
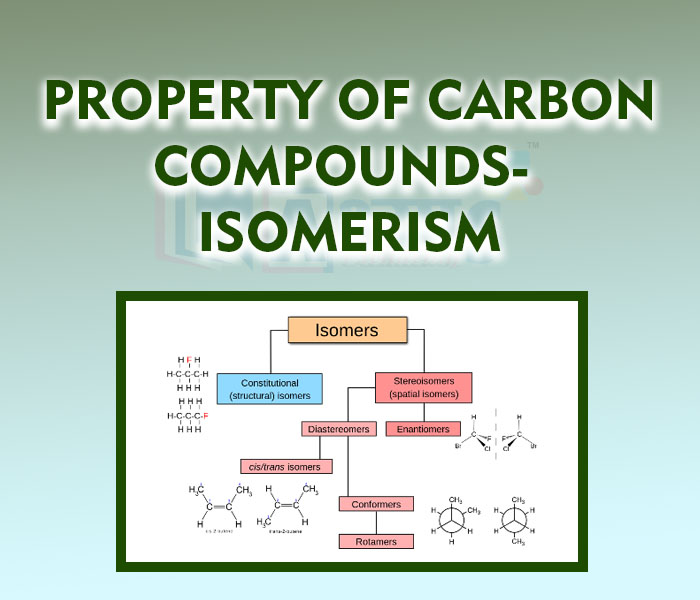
- Property of Carbon Compounds- Isomerism
Explore Concepts (Click & View)
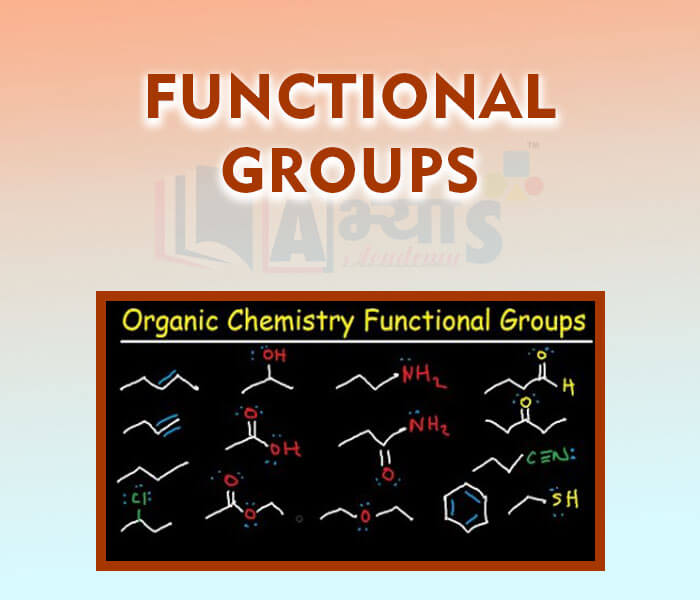
- Functional Groups
- Homologous Series

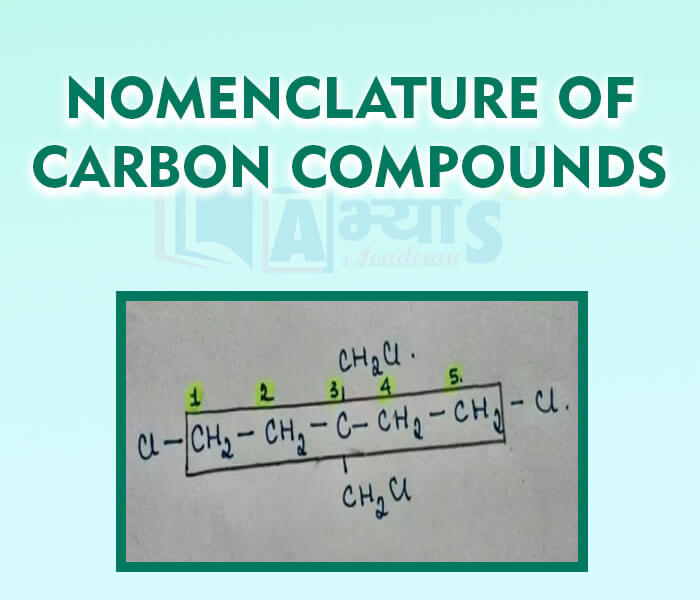
- Nomenclature of Carbon Compounds
- Combustion of Carbon Compounds

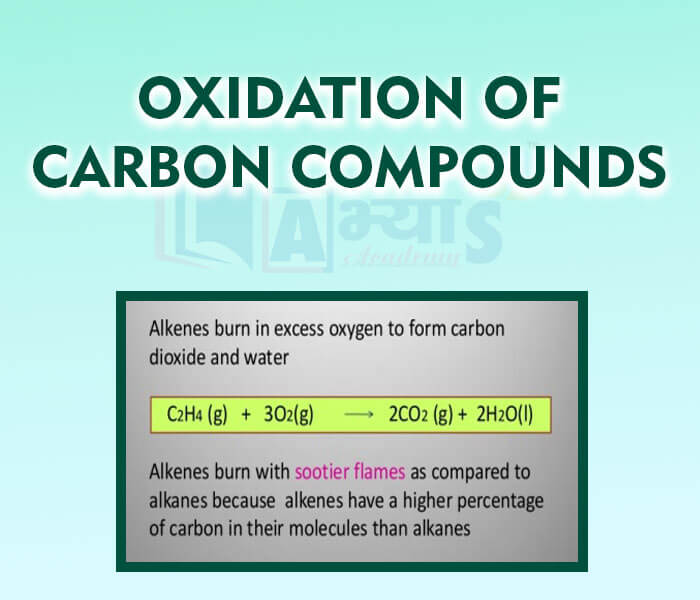
- Oxidation of Carbon Compounds
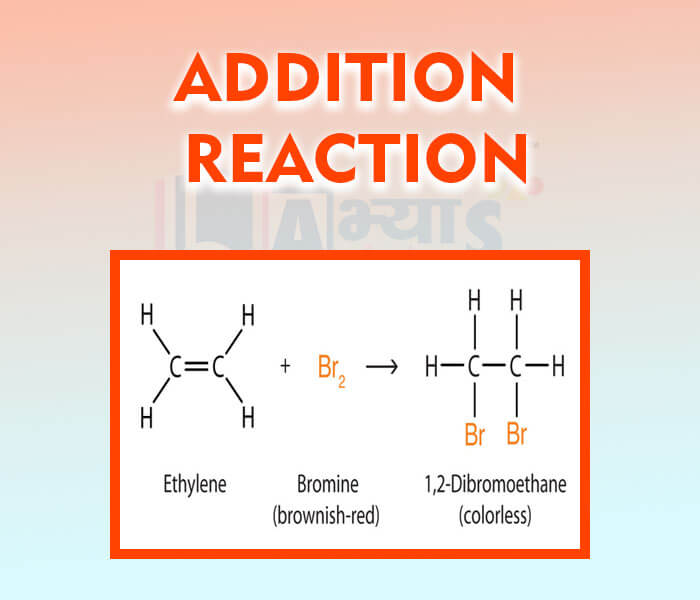
- Addition Reaction
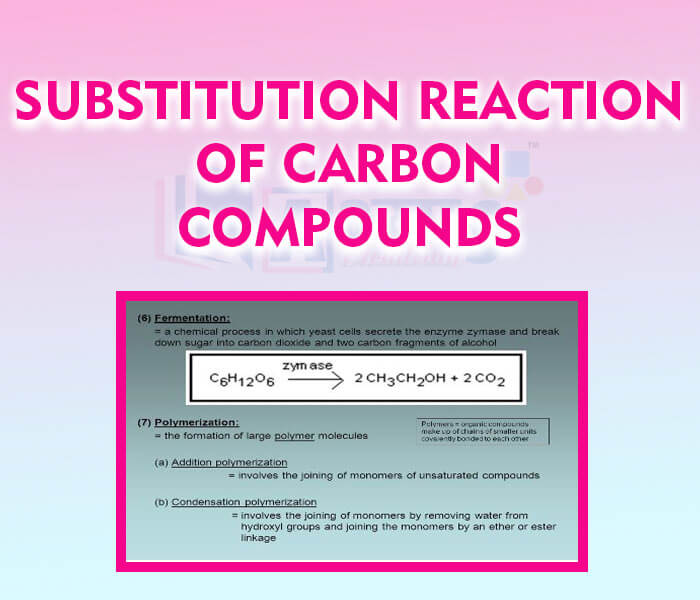
- Subsitution Reaction of Carbon Compounds
- Fossil Fuels

- Flames

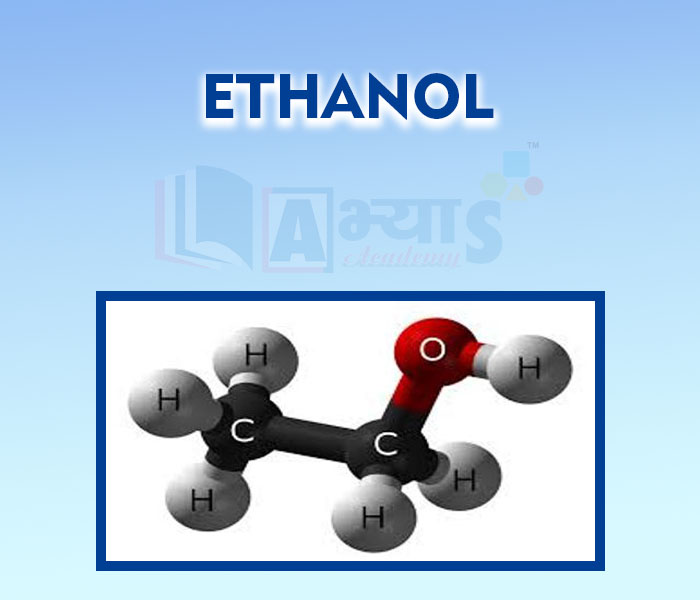
- Ethanol
- Effect of Ethanol on Human Beings

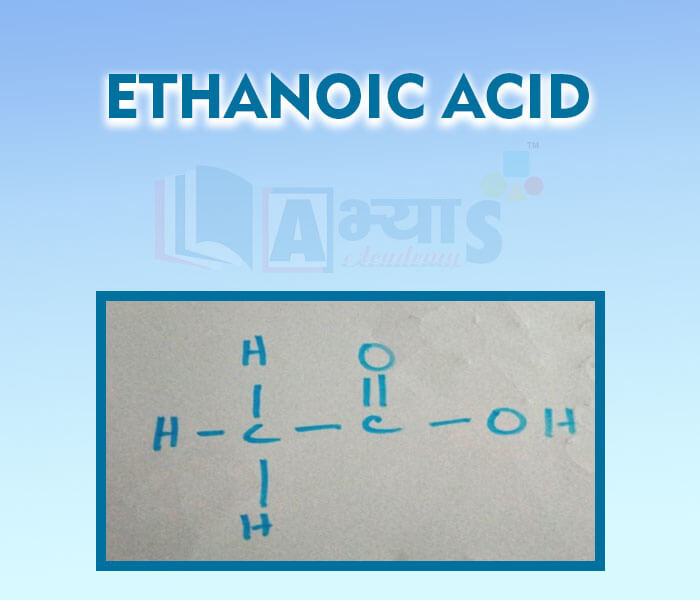
- Ethnoic Acid
- Esterification and Saponification

- Soaps and Detergents

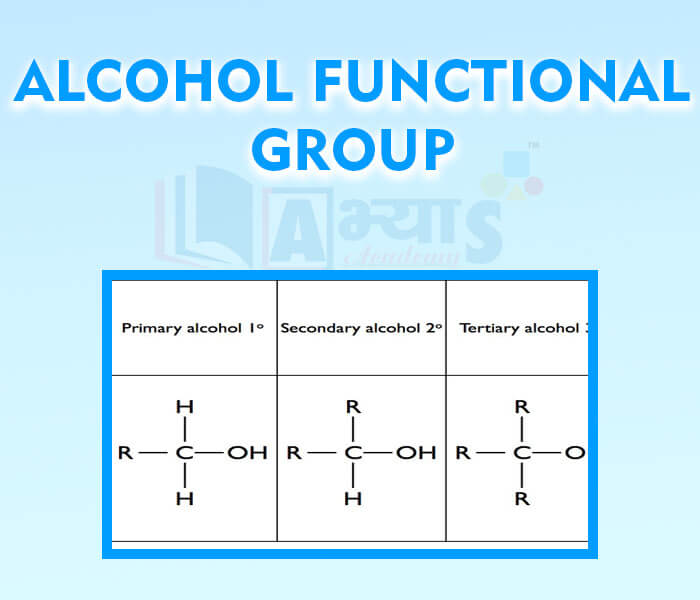
- Alcohol Functional Group
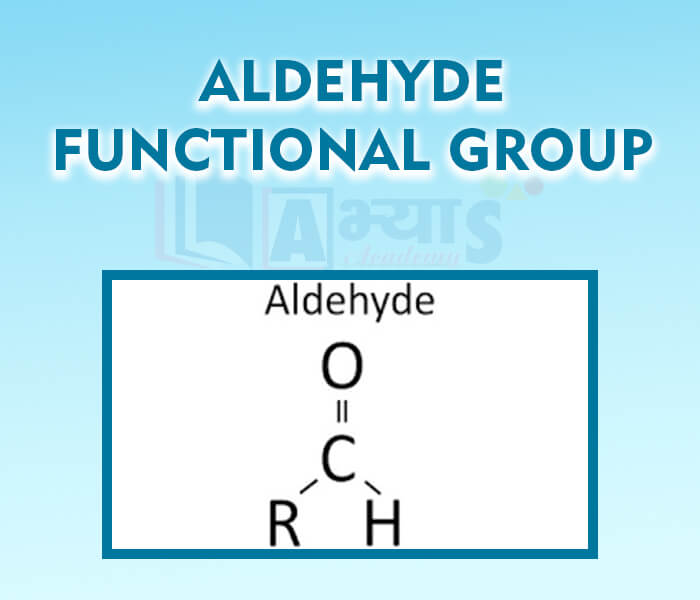
- Aldehyde Functional Group
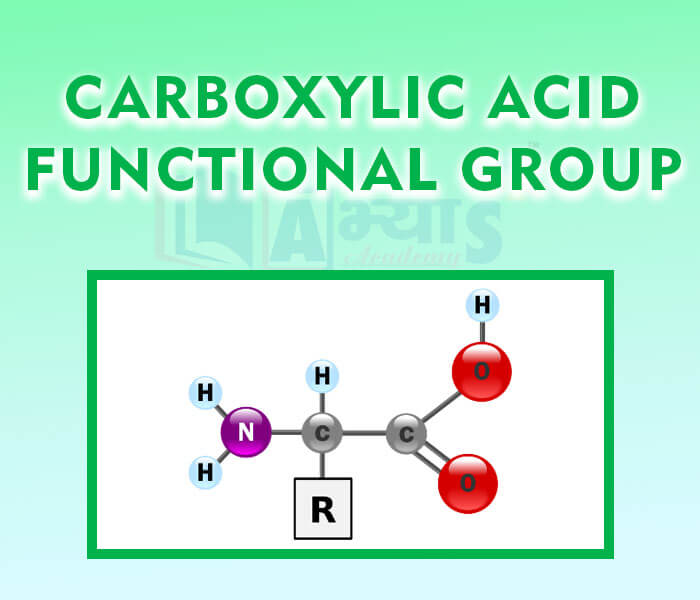
- Carboxylic Acid Functional Group
- Halogens Funcational Group
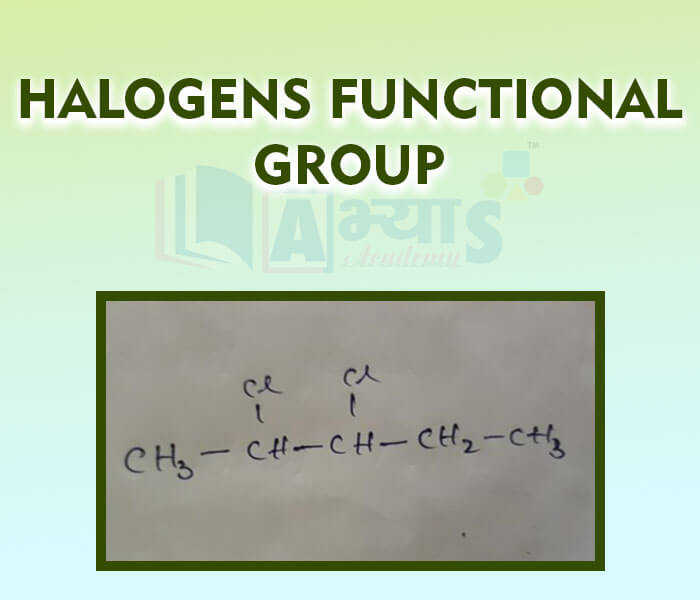
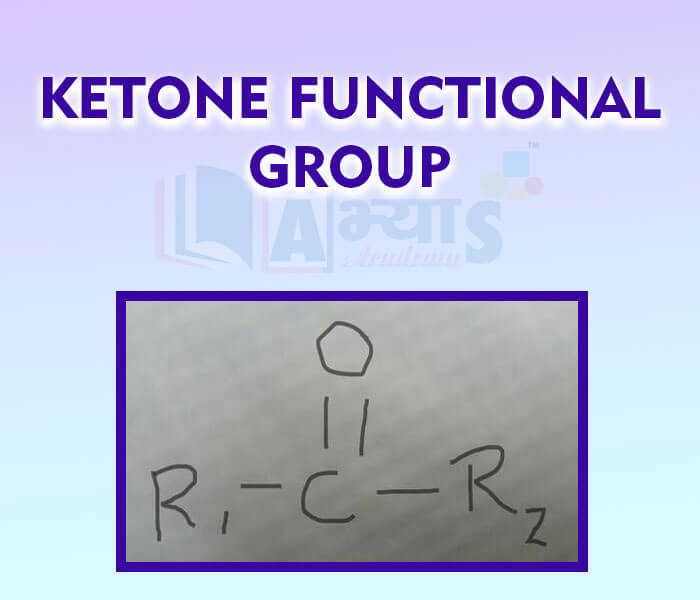
- Ketone Functional Group
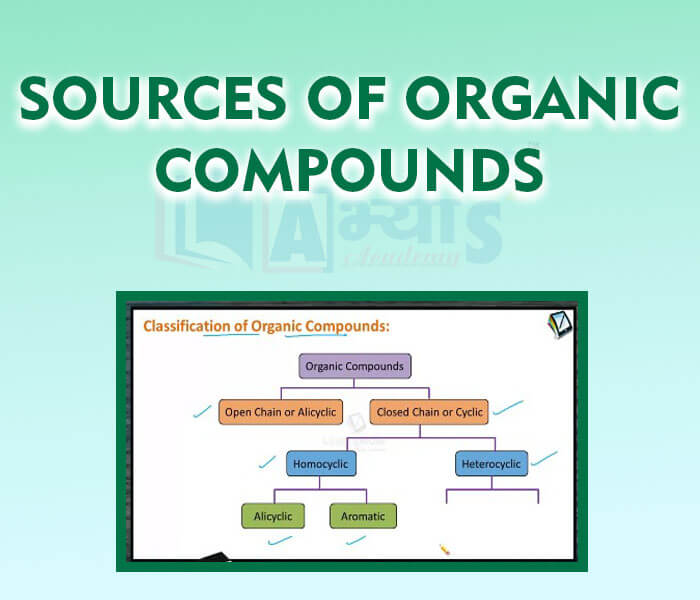
- Sources of Organic Compounds
Explore Concepts (Click & View)
- Earlier Attempts of Classification of Elements

- Dobreiner Triads

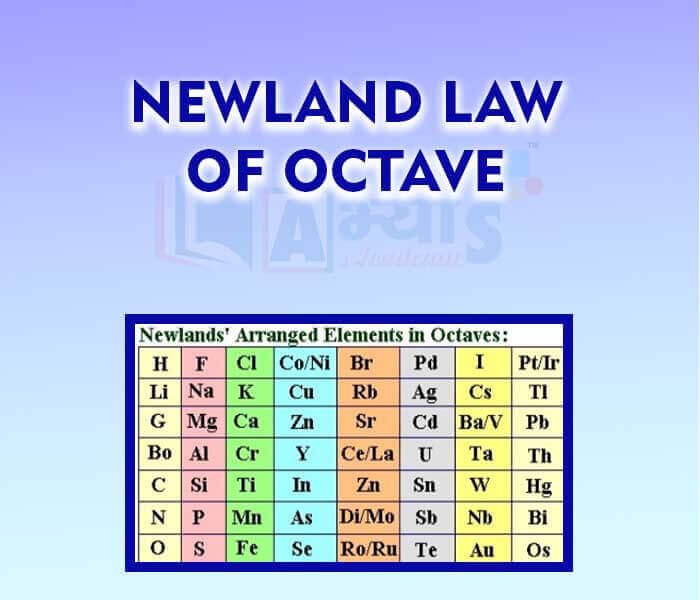
- Newland Law of Octave
- Mendeleev Periodic Table

- Achievements and Limitations of Mendeleev Periodic Table
Explore Concepts (Click & View)
- Modern Periodic Table

- Position of Elements in the Modern Periodic Table
- Trends in the Modern Periodic Table
- Metals and Non-Metals in Periodic Classification
Explore Concepts (Click & View)
- Renewable and NonRenewable Resources

- Management and Conservation of Natural Resources

- Sustainability of Natural Resources

- Ganga Action Plan

- The Chipko Movement

- Forest and Wild Life Resources

- Global Warming

- Greenhouse Effect

- Conservation of Soil and Water Resources

Explore Concepts (Click & View)
- Water As A Resource

- Big Dams

- Save The Narmada Movement

- The Adverse Effects of a Dam

- Harvesting of Water

- Watershed Management

- Coal and Petroleum

- Legal Measure to Save The Environment

Students / Parents Reviews [20]
About Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.

Shreya Shrivastava
8thAbhyas is good institution and a innovative institute also. It is a good platform of beginners.Due to Abhyas,he has got knoweledge about reasoning and confidence.My son has improved his vocabulary because of Abhyas.Teacher have very friendly atmosphere also.

Manish Kumar
10thAbhyas institute is one of the best coaching institute in the vicinity of Ambala Cantt area. The teachers of the institute are well experienced and very helpful in solving the problems of the students.The good thing of the institute is that it is providing extra classes for the students who are w...

Aman Kumar Shrivastava
10thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thIn terms of methodology I want to say that institute provides expert guidence and results oriented monitering supplements by requsite study material along with regular tests which help the students to improve their education skills.The techniques of providing education helps the students to asses...

Aman Kumar Shrivastava
10thAbhyas academy is great place to learn. I have learnt a lot here they have finished my fear of not answering.It has created a habit of self studying in me.The teachers here are very supportive and helpful. Earlier my maths and science was good but now it has been much better than before.

Barkha Arora
10thMy experience was very good with Abhyas academy. I am studying here from 6th class and I am satisfied by its results in my life. I improved a lot here ahead of school syllabus.

Ayan Ghosh
8thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thUsually we see institutes offering objective based learning which usually causes a lag behind in subjective examinations which is the pattern followed by schools. I think it is really a work of planning to make us students grab the advantages of modes of examination, Objective Subjective and Onli...

Anika Saxena
8thMy experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thMy experience with Abhyas academy is very nice or it can be said wonderful. I have been studying here from seven class. I have been completing my journey of three years. I am tinking that I should join Abhyas Academy in tenth class as I am seeing much improvement in Maths and English

Hridey Preet
9thThe experience was nice. I studied here for three years and saw a tremendous change in myself. I started liking subjects like English and SST which earlier I ran from. Extra knowledge gave me confidence to overcome competitive exams. One of the best institutes for secondary education.

Aman Kumar Shrivastava
10thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thBeing a parent, I saw my daughter improvement in her studies by seeing a good result in all day to day compititive exam TMO, NSO, IEO etc and as well as studies. I have got a fruitful result from my daughter.

Prisha Gupta
8thOne of the best institutes to develope a child interest in studies.Provides SST and English knowledge also unlike other institutes. Teachers are co operative and friendly online tests andPPT develope practical knowledge also.

Aman Kumar Shrivastava
10thAbhyas is an institute of high repute. Yogansh has taken admission last year. It creates abilities in child to prepare for competitive exams. Students are motivated by living prizes on basis of performance in Abhyas exams. He is satisfied with institute.

Yogansh Nyasi
7thIt was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.












